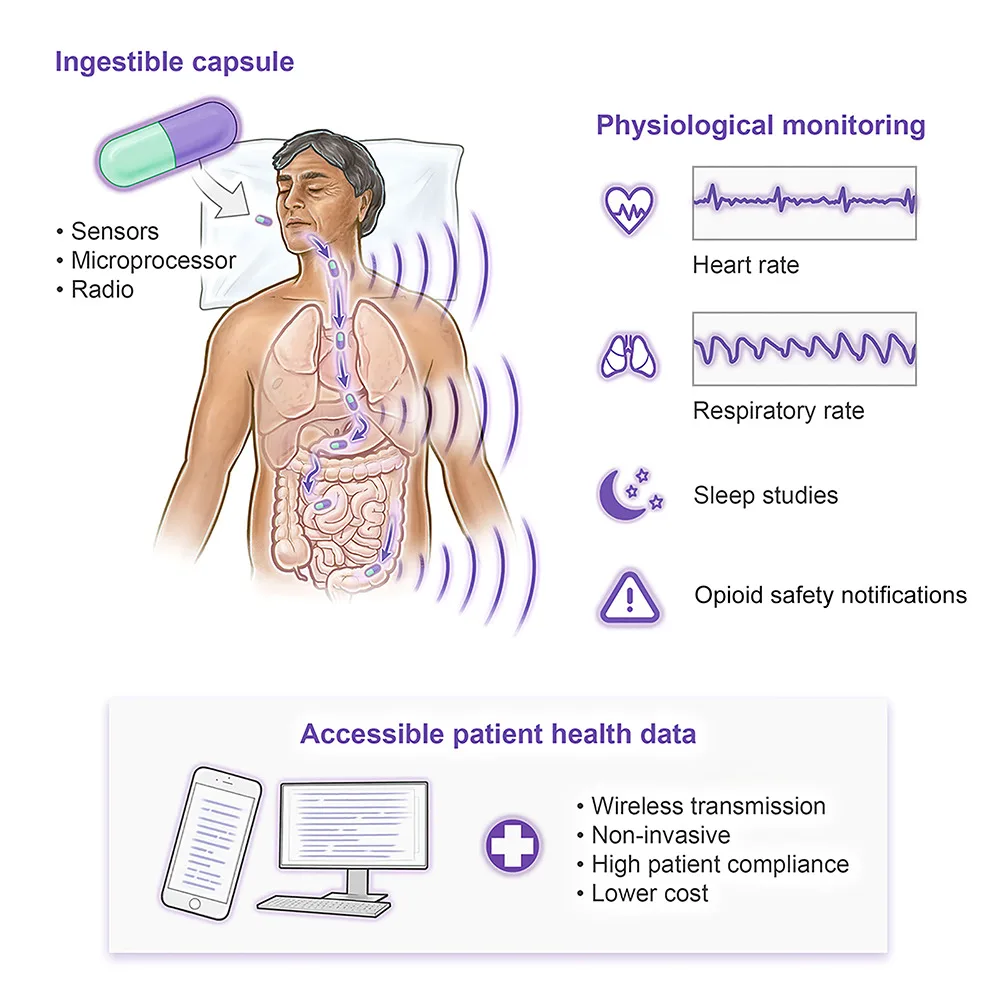
Device
First-in-Human Trial of an Ingestible Vitals-Monitoring Pill
DOI: https://doi.org/10.1016/j.device.2023.100125
Abstract
Ingestible electronics can transform how patients are diagnosed and treated across many conditions. We developed an ingestible vitals-monitoring pill (VM pill) capable of monitoring vital signs including respiratory rate and heart rate. VM pill performance was evaluated in a swine model of opiate overdose and in a human trial of patients in a sleep laboratory. Sleep studies involve admission to a facility, placement of multiple skin sensors, and overnight observation. We hypothesized that the VM pill could diagnose clinically significant changes in respiratory status, such as apnea, unobtrusively. The VM pill was evaluated in 10 human subjects with no adverse events. The data streams captured by the VM pill achieved high concordance with standard sleep study metrics. Ingestible vital sign monitors can transform the diagnosis of sleep-related respiratory disorders and can capture life-threatening events such as apnea or opioid overdose.